Submitted by Administrator on Mon, 07/09/2020 - 12:39
A new study from the Segal group uncovers the structural basis for intrinsic asymmetry in the spindle pathway under cyclin-dependent kinase control
Cell division gives rise to two progeny cells carrying copies of the progenitor's genetic material. In so call symmetric divisions, daughter cells also share the progenitor's identity. Yet, in the special case of asymmetric cell division, other cellular factors are transmitted unequally instructing daughters cells to adopt different fates. Stem cells may expand their pool through symmetric divisions. By contrast, stem cells dividing asymmetrically self-renew by producing two different progeny cells in a balanced manner - one retains the stem cell identity and potential for unlimited proliferation while the other embarks towards its terminal fate. This balanced choice is central to development and its perturbation may lead to cancer. One outstanding feature of many self-renewing stem-cell divisions is that they couple the polarised orientation of the mitotic spindle with an invariant pattern of age-dependent centrosome inheritance.
The Segal group studies the cell cycle control mechanisms for polarised spindle orientation in a cell dividing asymmetrically using baker's yeast as a model — the first organism to reveal an invariant pattern of spindle pole inheritance analogous to that later found in self-renewing stem cell divisions.
A new study, in collaboration with Sue Jaspersen's group at the Stowers Institute for Medical Research in the USA, demonstrates that age-dependent asymmetric spindle pole fate stems from structural asymmetry built into the spindle pole body duplication cycle (SPB, equivalent to the centrosome of animal cells) based on structured illumination microscopy and biochemical analyses. Moreover, the study shows that cyclin-dependent kinase governs the orderly sequence of events for new SPB assembly that effectively translates into the spatially and temporally regulated constellation of microtubule nucleation sites required for correct spindle morphogenesis and orientation. Given the significant conservation of cell cycle control elements and the microtubule nucleation machinery between yeast and man, this new study offers key insight into novel mechanisms for cell cycle control of protein complex assemblies with impact on differential centrosome inheritance.
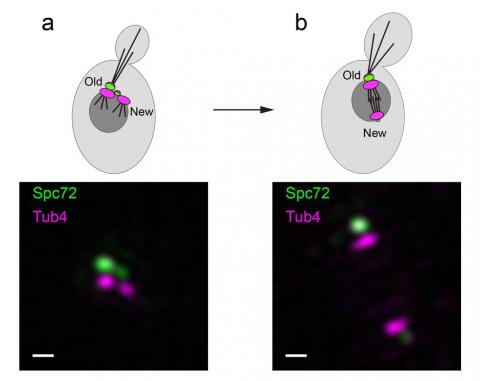
Figure: Structured illumination microscopy images corresponding to the stages depicted in the cartoons (a) duplicated side-by-side SPBs (b) spindle assembly. Asymmetric recruitment of Spc72 (green) under CDK control translates into the absence of microtubule nucleation factors at the new SPB cytoplasmic face. By virtue of this distinction, only the old SPB is competent to organise astral microtubules at onset of spindle assembly, thus priming its inheritance by the bud [Bar, 200 nm].
