Submitted by Christine Alexander on Fri, 05/10/2018 - 09:43
Research published in Nature online this week [1] describes the remarkable organisation of ‘gastruloids’, small aggregates of mouse Pluripotent Stem Cells that, when grown in the lab, develop a spatial and temporal organization of gene expression that mirrors much of what happens in early embryos [2]. The work is the result of a collaboration between the Department of Genetics and two swiss institutions, the University of Geneva and the Ecole Polytechnique Federal de Lausanne (EPFL).
The research in the Department of Genetics was undertaken by Naomi Moris, David Turner and Peter Baillie-Johnson in the laboratory of Alfonso Martinez Arias which has a long-standing interest in understanding how cells make decisions during the development of an embryo. Particularly, the lab studies how the outcome of these decisions influences the organization of cells in space and time to generate the organs and tissues that make up an organism, in this case a mouse.
A few years ago, Susanne van den Brink (now a graduate student at the Hubrecht Institute in The Netherlands), together with David Turner and Peter Baillie-Johnson, found a way to coax mouse embryonic stem cells (ESCs) to form small aggregates which exhibited a high degree of organization [2-5]. Surprisingly, these events would only happen if a small, but defined, number of cells were to come together under defined culture conditions. The organisation and the movement of the cells in these aggregates resembled events during a process known as gastrulation, when the cells of the embryo become organised into 3 layers, and this is why these structures were called gastruloids. The structures exhibited a polarization with hints of an anteroposterior axis. However, gastruloids are not embryos, principally because they lack a brain and therefore cannot give rise to a full organism. Importantly, they also undergo these events in the absence of any extraembryonic tissues; this was surprising as these tissues are thought to provide valuable information to pattern the embryo.
The latest research [1], performed in collaboration with the laboratories of Denis Duboule and Matthias Lutolf in Geneva and Lausanne, extends the culture of gastruloids to a more mature state and compares their dynamics directly to mouse embryos. Surprisingly, the study finds that these mature gastruloids display the primordia for various organs and tissues, organized with regard to the three body axes that characterize an organism (anteroposterior, dorsovental and mediolateral) and exhibit a close similarity to the developing mouse embryos. Gastruloid display all mesodermal derivatives (that give rise to the heart, kidney, muscles, bone and spinal cord) which, in the gastruloids, are organized along a similar axis as in the embryos. The gastruloids even appear to have a node-like structure, an important embryonic feature that is important in patterning the left and right sides of an organism i.e. allows the positioning of the heart, the liver and the pancreas. One remarkable observation in this work concerns the expression of a group of genes, the Hox genes, which in the embryo are expressed sequentially, from head to tail, in a manner that mirrors their organization in the chromosome. In the embryo, Hox genes are expressed in a tightly ordered and regulated temporal order. Gastruloids mimicked this spatiotemporal organization of Hox gene expression and could be used to further explore the dynamic regulation of gene expression.
“We’re just beginning to understand the power of this gastruloid system” says Naomi Moris, who is a first co-author of the work. “What we have found is incredibly interesting and we hope the system could be used to help us understand the fundamental principles of gene regulation in early embryo development”
The organised complexity is very surprising and suggests that gastruloids can become an extremely useful tool to study development ex vivo, making a big contribution to the 3Rs (Reduction, Refinement and Replacement in animal research). David Turner, co-author of the work, is now a NC3R David Sainsbury Research fellow, and sees the very real potential to impact the 3Rs. “The fact that these gastruloids show asymmetries in their left-right axis allows us to study laterality defects without animals, which is something I am interested in and which we couldn’t do easily before Gastruloids; importantly this system allows us to significantly reduce and replace animals for this work for this and related studies.”
“In some ways this might be the beginning of a long story’ says Alfonso Martinez Arias, who adds “we have been surprised by how well organized gastruloids are and, while they are not embryos, and it is important to emphasize this, they have so many embryo-like features. Two details that have surprised us are the fact that this complexity arises without extraembryonic tissues and that, despite the amazing spatial and temporal organization of the transcriptional programs that they exhibit, the organization of the cells is messy, they are not well organized into tissues suggesting a surprising disconnect between the activities of genes and the activities of cells, which could not have been anticipated. In this regard, Peter Baillie-Johnson, now in the Stem Cell Institute in Cambridge, is using gastruloids to explore the mechanisms of gastrulation’.
The work, and the earlier related publications of the group [2-5], suggests and establishes gastruloids as potential new experimental system for mammalian development though, as Martinez Arias says “only time will tell. One thing we are confident about is that the system is reproducible and that several groups have adopted it already. The work is also an example of cooperation and interactions between Institutions across Europe, which have provided such a fruitful opportunity to share knowledge and research.”
Contact: Alfonso Martinez Arias (ama11@hermes.cam.ac.uk) and Denis Duboule (Denis.Duboule@unige.ch)
Image information
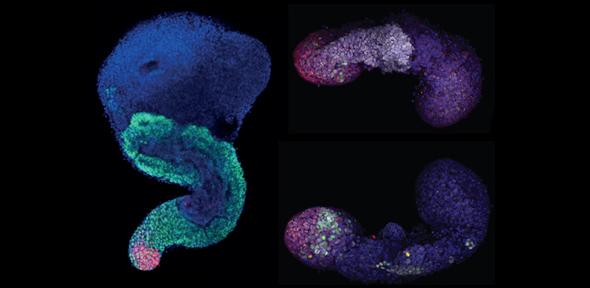
Top of page : A seven day old gastruloid (left) and two views of a five day gastruloid. The 7 day old shows neural precursors (green) distributed along the antero-posterior axis (top to bottom); cells marked in magenta are reminiscent of the embryonic tail and indicate the direction of elongation. Picture produced by Mehmet Girgin Ugur (laboratory of Matthias Lutolf, EPFL, Lausanne, Switzerland). The five day old shows a dorsal view (top) with neural tissue (white) and the tail-like region (red) and a ventral view (bottom) with the tail region (red) and the expression of a gene call Nodal, which as you can see is only expressed on one side (credit for this to David Turner)
Home page : A collection of 144hrs gastruloids
>> Click here to see movie of gastruloid development
>> Click here to see David Turner video
References
1. Beccari, L., et al., Multiaxial self organization properties of mouse embryonic stem cells into gastruloids. Nature, 2018. doi:10.1038/s41586-018-0578-0, 2018.
2. Baillie-Johnson, P., et al., Generation of aggregates of mouse ES cells that show symmetry breaking, polarisation and emergent collective behaviour. JOVE, 2014. doi: 10.3791/53252(105).
3. Turner, DA., et al., Gastruloids develop the three body axes in the absence of extraembryonic tissues and spatially localised signalling. bioRxiv, 2017. doi.org/10.1101/104539
4. Turner, D.A., et al., Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development, 2017. 144(21): p. 3894-3906.
5. Turner, D.A., et al., Wnt/beta-catenin and FGF signalling direct the specification and maintenance of a neuromesodermal axial progenitor in ensembles of mouse embryonic stem cells. Development, 2014. 141(22): p. 4243-53.
Read more
>> Report on University of Cambridge news
>> University of Geneva press release
>> David Turner talks about the discovery on the NC3Rs website
>> Peter Baillie-Johnson and David Turner talk about their earlier research on gastruloids
