The course is organised into 5 modules from which you select 4, ensuring that you acquire the necessary breadth of background in the subject. Each module is made up of ~24 lectures. The modules aim to cover the range of genetics from cellular to organism level and will show how the latest developments (in areas such as sequencing technologies and genome assembly, functional and computational biology) are being applied to the problems of how genes in different species are organised, expressed and interact, to give the final phenotype.
Michaelmas Term (2/2) – no options:
- Module 1: Genomes
- Module 2: Early Development & Patterning: Genetic & Cellular Mechanisms
Lent Term (2/3) – you select 2 modules out of:
- Module 3: Genetics of Health & Disease
- Module 4: Evolutionary Genetics & Adaptation
- Module 5: Mathematical Genetics
Module 1 : Genomes (MT)
This module will first explore how eukaryotic genomes are organised. Starting at the level of the DNA sequence, it will examine how genomes are sequenced, the sequence assembled and how sequence-based technologies, like Hi-C, reveal organisation at the chromosome level. The make-up of genomes, from genes and their regulatory elements, to the importance of genetic variation, and of the non-coding genome and repeat DNAs, will be considered. We will introduce the genomics approaches that underpin the functional analysis of genomes, including technologies for measuring gene expression, transcriptional activity and chromatin states. We will look at the organisation of the chromatin fibre and compare eukaryotic chromosomes in interphase and M phase, considering mechanisms for compaction and examining key functional elements like the centromere and telomere. The epigenome will be discussed including its role in gene regulation and the silencing of transposable elements. The importance of maintaining genome stability and the impact of aneuploidy and polyploidy, along with characteristics of the cancer genome, will be considered. This will lead to an exploration of the control mechanisms that promote correct cell cycle progression and the accurate segregation of genes and chromosomes into daughter cells at cell division, centred on key cell cycle protein kinases, phosphatases and checkpoints.
Module 2 : Early Development & Patterning: Genetic & Cellular Mechanisms (MT)

This module will look at:
- Early embryo development
- How animals’ body plans are formed
- Gene regulatory & signalling interactions
- Dynamic cell behaviours & morphogenesis
You will therefore learn about the key principles of embryonic development, taking examples from a range of early developmental events, such as cell fate determination, germline development, gastrulation, segmentation, and somitogenesis, in both invertebrate and vertebrate systems. During the course of the module you will be introduced to a range of modern techniques applicable to the study of development including molecular, genetic and imaging technologies.
Module 3 : Genetics of Health & Disease (LT)
This module will be human-centric and, while it will look at some specific diseases, it will focus more on genetic approaches to understanding disease mechanisms and developing therapies, rather than on shallower coverage of an extensive list of diseases. The module will begin with understanding human mutation, and the genetic approaches to both monogenic and multigenic diseases. Monogenic diseases include the growing number of rare diseases emerging from genome sequencing in the clinic. While individually rare, they are collectively quite common, and can be informative about the basic biology and disease mechanisms of more common diseases. Multigenic diseases include many common conditions with a genetic component and we will address approaches to identifying the causative genes, such as genome-wide association studies, and how they help us to understand the disease. Implications for personalised medicine are also discussed, as are the prospects and uses for gene therapy. This module will look at diseases for which genetic approaches have revealed much about their mechanisms, and potentially also therapies – neurodegenerative diseases (such as Parkinson’s or Alzheimer’s), mitochondrial disorders, genomic imprinting disorders and cancer. Finally, infectious disease, including the dynamics and genomics of viral and bacterial pathogens, and antimicrobial resistance will be examined.
Module 4 : Evolutionary Genetics & Adaptation (LT)

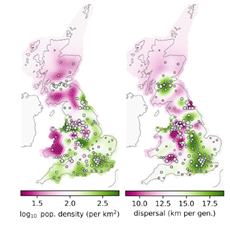
Modern evolutionary theory has its roots in the union of Mendelian genetics with Darwin’s theory of evolution, two of the great unifying themes of biology. This course will consider the process of evolution from a genetic perspective, exploring the central topics of natural selection, adaptation and genetic drift, and combining a variety of empirical and theoretical approaches. Alongside this, the course will explore how genomes themselves are shaped by selection, drift and their evolutionary history. The first half of the module will explore the genetic basis of adaptation. Do we expect evolutionary change to involve few or many genes, and how might we go about identifying the genes underlying a particular trait? What kinds of genes control evolutionary changes in morphological traits? We look at the genes underlying convergent evolution as a way of understanding the predictability of evolutionary change. Genomic data contain a wealth of information about the history of populations and natural selection, and population genetics provides a framework to reconstruct these processes. The second half of the module will look at the evolution of genomes and conflict within genomes. We will begin by examining the evolution of key features of genomes – sex chromosomes, introns, repetitive DNA, gene expression and mutation rates. We will finish by considering one of the conundrums of evolutionary biology – why some species reproduce sexually – from a theoretical and empirical perspective.
Module 5 : Mathematical Genetics (LT)
The aim of this module will be to give students a thorough grounding in mathematical approaches to the study of genetics. Emphasis will be placed on fundamental principles, equipping students with a deeper understanding of the various roles of mathematics in the field, and the ability to understand and even develop the next generation of methods and tools. The material covered will include the nature and uses of mathematical models, including latent variable models, and statistical methods of inference. The course will also cover topics relevant to particular areas of genetics, including evolutionary, developmental, and biophysical genetics, as well as other subfields that link genotype to phenotype. These topics will include mathematical population genetics (including the coalescent approach, which underpins inferences from genomic sequence data); quantitative genetics (the inheritance, architecture and evolution of complex and continuously-varying traits); dimensionality reduction approaches, which are essential to understanding large data sets (e.g. single-cell data), and dynamical systems modelling of gene regulatory networks.
Page updated 31/07/2025