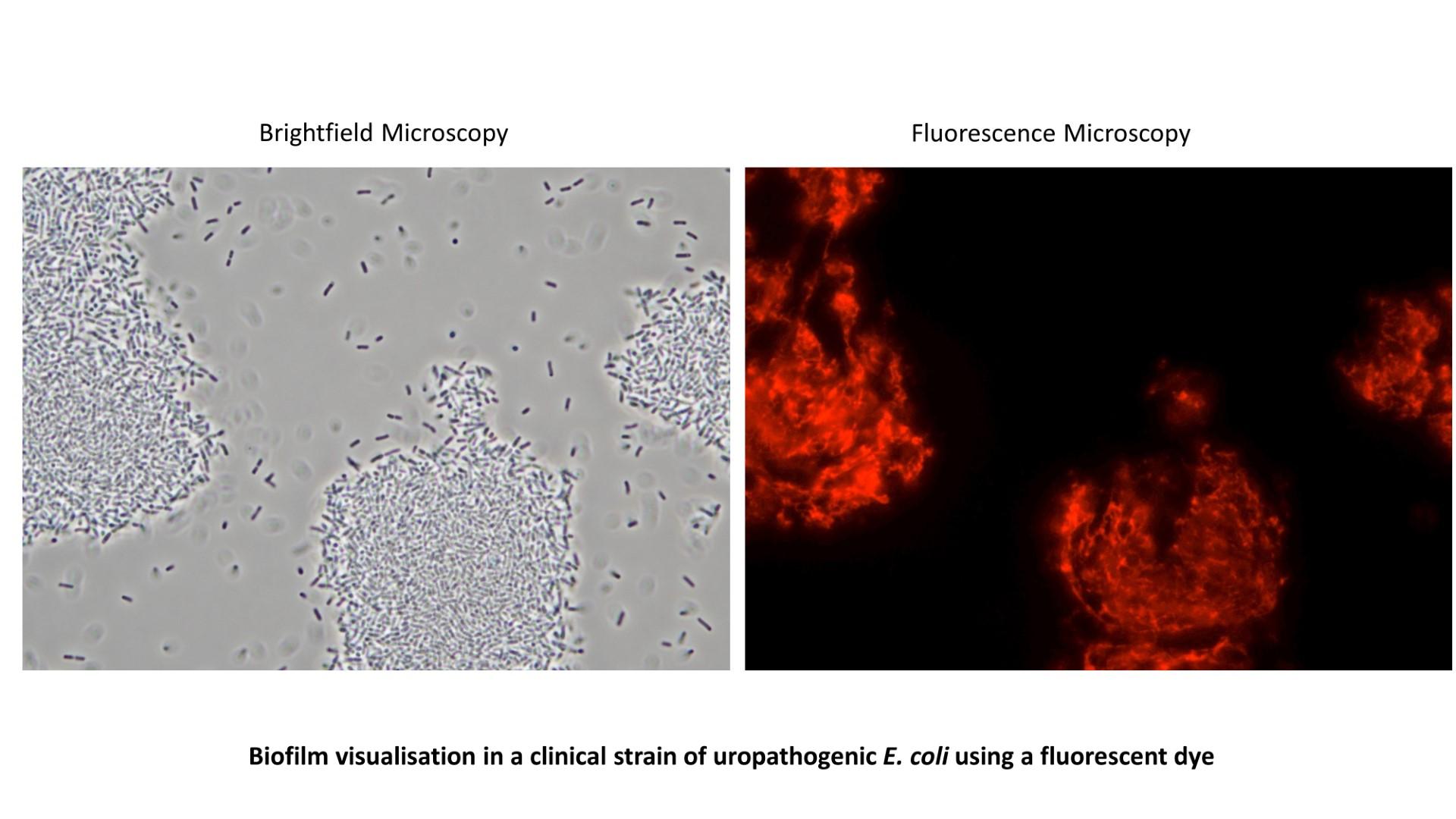
The role of biofilms and persisters in bacterial infections
Keywords
Antibiotic, biofilm, persisters, UTIs
Research Interests
Research in the Zarkan laboratory is focused on understanding and combatting bacterial biofilms and antibiotic persisters, using E. coli as a model organism. Biofilms and persisters are two important aspects of antibiotic resistance. Biofilms provide structural protection for bacteria and other microorganisms against antimicrobials and the host immune system, while persistence refer to the ability of subpopulation of genetically sensitive bacteria to survive antibiotic treatment by virtue of their dormancy. Biofilms and persisters are associated clinically with treatment failure and recurrent infections. In the case of E. coli, biofilms and persisters are common in patients with urinary tract infections (UTIs) leading to recurrent UTIs. We aim to address the following questions:
- What is the role of the bacterial PLP-dependent enzymes in the formation of biofilms and persisters?
- What is the relation between bacterial membrane potential and antibiotic persistence?
- How do urinary extracellular vesicles contribute to combatting biofilms and persisters in UTIs?
