Submitted by Lottie Groocock on Wed, 05/04/2023 - 14:15
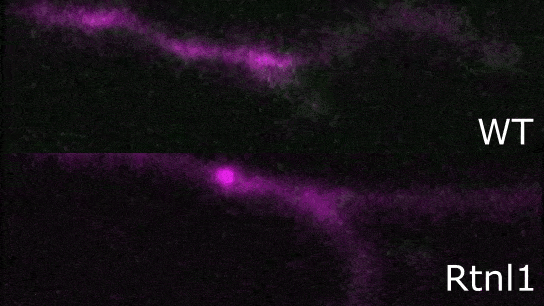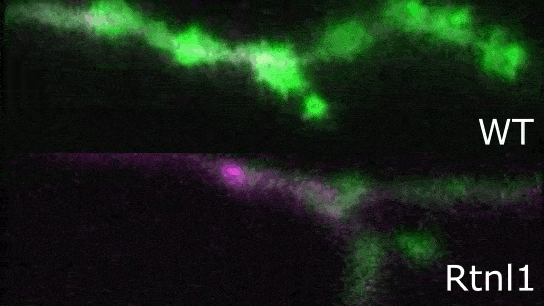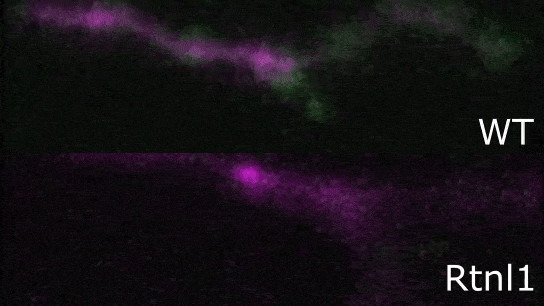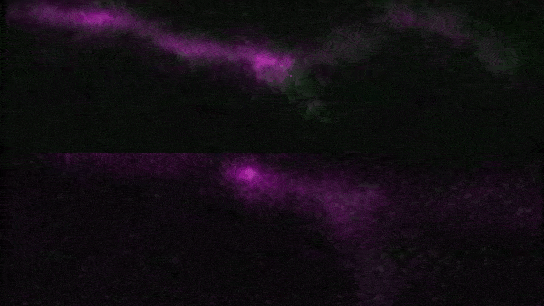
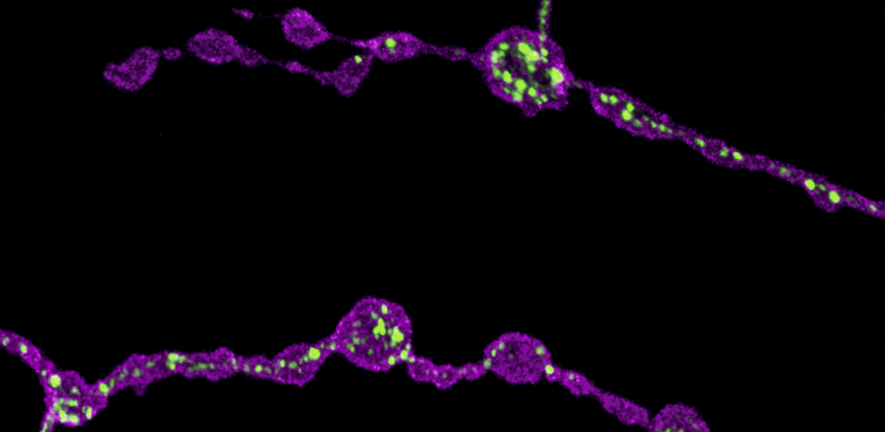
Image: Confocal image showing presynaptic terminals from motor neurons of Drosophila larva. Endoplasmic reticulum (ER) lumen is labelled with GFP::HDEL (green) and plasma membrane is labelled with CD4::tdTom (magenta). Loss of function of Rtnl1 leads to a loss of ER tubules while keeping the regions of the network with higher luminal space (i.e., cisternae).
Endoplasmic reticulum defects impair the function of motor neuron terminals
In March 2023, Researchers from the O'Kane group published their paper 'Drosophila SPG12 ortholog, reticulon-like 1, governs presynaptic ER organization and Ca2+ dynamics' in the Journal of Cell Biology (link here)
"We studied a Drosophila model of hereditary spastic paraplegia (HSP), and tested its impact on presynaptic endoplasmic reticulum organization and Ca2+ dynamics. For our model, we used Rtnl1 mutants, which are orthologs of human SPG12, a known HSP gene. In these mutants, we observed that a specific reduction of ER surface (tubulation) leads to lower Ca2+ fluxes in presynaptic compartments (ER lumen, mitochondria, and cytosol), which was quite surprising, as we did not see an overall reduction in ER volume. We found that these defects are dependent on the levels of STIM protein (involved in Ca2+ exchange at ER-plasma membrane contact sites), appear in both tonic and phasic motor neurons, and correlate with decreased synaptic function. Our results suggest mechanisms whereby altered organization of ER tubules might control synapse function and cause hereditary spastic paraplegia (HSP)."
The clip below shows both wildtype and mutant synaptic activity.